Carbon nanotubes are the most successful materials that are now attracting a broad range of scientists and industries due to their fascinating physical and chemical properties. In this post, we will take you through the structure, synthesis and the most important applications of carbon nanotubes in different fields.
Introduction
Carbon nanotubes, long thin cylinders of carbon, were discovered in 1991 by Iijima. Carbon nanotubes (CNTs) are allotropes of carbon which are members of the fullerene structural family, which also includes the spherical buckyballs. These are large macromolecules which are unique for their size, shape and remarkable physical properties.
The nature of the bonding of a nanotube is described by applied quantum chemistry, specifically, orbital hybridization. The chemical bonding of nanotubes is composed of sp2 bonds, similar to those of graphite. This bonding structure, which is stronger than the sp3 bonds found in diamond, provides the molecules with their unique strength. Nanotubes naturally align themselves into “ropes” held together by Van der Waals forces. Under high pressure, nanotubes can merge together, trading some sp² bonds for sp³ bonds, giving the possibility of producing strong, unlimited-length wires through high-pressure nanotube linking.
History:
The discovery that carbon could form stable, ordered structures other than graphite and diamond stimulated researchers worldwide to search for other new forms of carbon. The search was given new impetus when it was shown in 1990 that carbon-60(buckminister fullerence) could be produced in a simple arc-evaporation apparatus readily available in all laboratories. It was using such a evaporator that the Japanese scientist Sumio Iijima discovered fullerence-related carbon nanotubes in 1991. The tubes contained atleast two layers, often many more, and ranged in outer diameter from about 3nm to 30nm.
In 1993, a new class of carbon nanotubes was discovered, with just a single layer. These single-walled nanotubes are generally narrower than the multiwalled tubes, with diameters typically in the range 1-2 nm, and tend to be curved rather than straight. It was soon established that these new fibers had a range of exceptional properties, and this sparked off an explosion of research in to carbon nanotubes. It is important to note, however, that nano scale tubes of carbon produced catalytically, had been known for many years before Iijimas discovery. The main reason why these early tubes did not excite wide interest is that they were structurally rarther imperfect, so did not have particularly interesting properties. Recent reseach has focused on improving the quality of catalytically-produced nanotubes.
Structure:
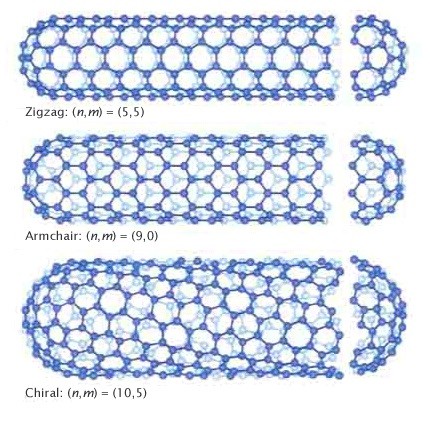
The bonding in carbon nanotubes is sp², with each atom joined to three neighbours, as in graphite. The tubes can therefore be considered as rolled-up graphene sheets (graphene is an individual graphite layer). There are three distinct ways in which a graphene sheet can be rolled into a tube, as shown below.
The first two of these, known as armchair and zig-zag have a high degree of symmetry. The terms “armchair” and “zig-zag” refer to the arrangement of hexagons around the circumference. The third class of tube, which in practice is the most common, is known as chiral, meaning that it can exist in two mirror-related forms.
Types of CarbonNanotubes
a) SINGLE-WALLED CNTs
Most single-walled nanotubes(SWNT) have a diameter close to 1nm, with a tube length that can be many thousands of times longer. SWNTs are very important carbon nanotube because they exhibit important electric properties that are not shared by the multi-walled carbon nanotubes(MWNT) varients.
SWNTs can be excellent conductors and the most building block of SWNT system is the electic wires. One useful application of SWNTs is in the development of the first intramolecular field effect transistors(FETs).
b) MULTI-WALLED CNTs: Multi-walled nanotubes (MWNT) consist of multiple rolled in on themselves to form a tube shape. There are two models which can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled up newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 0.33 nm.
c) FULLERITE: Fullerites are the solid-state manifestation of fullerences and related compounds and materials. Being highly incompressible nanotube forms, polymerized single-walled nanotubes (P-SWNT) are a class of fullerites and are comparable to diamond in terms of hardness.
SYNTHESIS
a) ARC DISCHARGE METHOD
Nanotubes were observed in 1991 in the carbon soot of graphite electrodes during an arc discharge, by using a current of 100 amperes, that was intended to produce fullerenes. However, the first macroscopic production of carbon nanotubes was made in 1992 by two researchers at NEC’s Fundamental Research Laboratory at France. The method used was the same as in 1991. During this process, the carbon contained in the negative electrode sublimates because of the high temperatures caused by the discharge. Because nanotubes were initially discovered using this technique, it has been the most widely used method of nanotube synthesis.
The yield for this method is up to 30 percent by weight and it produces both single- and multi-walled nanotubes with lengths of up to 50 micrometres.
b) LASER ABLATION PROCESS
In the laser ablation process, a pulsed laser vaporizes a graphite target in a high-temperature reactor while an inert gas is bled into the chamber. The nanotubes develop on the cooler surfaces of the reactor, as the vaporized carbon condenses. A water-cooled surface may be included in the system to collect the nanotubes.
It was invented by Richard Smalley and co-workers at Rice University, who at the time of the discovery of carbon nanotubes, were blasting metals with the laser to produce various metal molecules. When they heard of the discovery they substituted the metals with graphite to create multi-walled carbon nanotubes. Later that year the team used a composite of graphite and metal catalyst particles to synthesise single-walled carbon nanotubes.
This method has a yield of around 70% and produces primarily single-walled carbon nanotubes with a controllable diameter determined by the reaction temperature.
c)CHEMICAL VAPOUR DEPOSITION
 The catalytic vapor phase deposition of carbon was first reported in 1959, but it was not until 1993 that carbon nanotubes could be formed by this process. In 2007, researchers at the University of Cincinnati (UC) developed a process to grow 18 mm long aligned carbon nanotube arrays.
The catalytic vapor phase deposition of carbon was first reported in 1959, but it was not until 1993 that carbon nanotubes could be formed by this process. In 2007, researchers at the University of Cincinnati (UC) developed a process to grow 18 mm long aligned carbon nanotube arrays.
uring CVD, a substrate is prepared with a layer of metal catalyst particles, most commonly nickel, cobalt, iron, or a combination. The diameters of the nanotubes that are to be grown are related to the size of the metal particles. This can be controlled by patterned deposition of the metal, annealing, or by plasma etching of a metal layer. The substrate is heated to approximately 700°C. To initiate the growth of nanotubes, two gases are bled into the reactor: a process gas (such as ammonia, nitrogen, hydrogen, etc.) and a carbon-containing gas (such as acetylene, ethylene, ethanol, methane, etc.). Nanotubes grow at the sites of the metal catalyst; the carbon-containing gas is broken apart at the surface of the catalyst particle, and the carbon is transported to the edges of the particle, where it forms the nanotubes as shown in the figure above.
CVD is a common method for the commercial production of carbon nanotubes.. For this purpose, the metal nanoparticles will be carefully mixed with a catalyst support (e.g., MgO, Al2O3, etc) to increase the specific surface area for higher yield of the catalytic reaction of the carbon feedstock with the metal particles. One issue in this synthesis route is the removal of the catalyst support via an acid treatment, which sometimes could destroy the original structure of the carbon nanotubes. However, alternative catalyst supports that are soluble in water have been shown to be effective for nanotube growth. If a plasma is generated by the application of a strong electric field during the growth process (plasma enhanced chemical vapor deposition), then the nanotube growth will follow the direction of the electric field. By properly adjusting the geometry of the reactor it is possible to synthesize vertically aligned carbon nanotubes.
In 2007, a team from Meijo University has shown a high-efficiency CVD technique for growing carbon nanotubes from camphor. A team of researchers at Rice University, until recently led by the late Dr. Richard Smalley, has concentrated upon finding methods to produce large, pure amounts of particular types of nanotubes.
CVD growth of multi-walled nanotubes is used by several companies to produce materials on the tonne scale, including NanoLab Bayer, Arkema, Nanocyl, Nanothinx, Hyperion Catalysis, Mitsui, and Showa Denko.
Properties:
The most important properties of CNTs are
a) Strength: CNTs are the strongest and stiffest materials on earth, in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp² bonds formed between individual carbon atoms.
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional or bending stress.
b) Thermal: All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as ballistic conduction, but good insulators laterally to the tube axis. The temperature stability of carbon nanotubes is established to be up to 2800 degrees Celsius in vacuum and about 750 degrees Celsius in air.
c) Chemical Reactivity: The chemical reactivity of a CNT is, compared with a graphitesheet, enhanced as a direct result of the curvature of the CNT surface.
d) Electrical Conductivity: Depending on their chiral vector, carbon nanotubes with a small diameter are either semi-conducting or metallic.
APPLICATIONS
a) Structural
Concrete: In concrete, they increase the tensile strength, and halt crack propagation.
Polyethylene: Researchers have found that adding them to polyethylene increases the polymer’s elastic modulus by 30%.
Sports equipment: CNTs are used in different sports equipments such as tennis rackets, bike parts (racing bikes), golf balls etc.
Space Elevators: This will be possible only if tensile strengths of more than about 70 GPa can be achieved. Monoatomic oxygen in the Earth’s upper atmosphere would erode carbon nanotubes at some altitudes, so a space elevator constructed of nanotubes would need to be protected (by some kind of coating). Carbon nanotubes in other applications would generally not need such surface protection.
Others: Bridges, clothes, combat jackets, ultrahigh-speed flywheels etc.
Conclusion:
Rise in demand and production, and ease of accessibility of carbon nanotubes would lead to the extensive use of carbon nanotubes in a wide variety of applications. The use of nanotechnology for human will become common need in 21st century. As world is suffering from serious pollution problems, hydrogen will becoming need of 21st century & carbon nanotubes provide better solution for hydrogen storage.
Nanotubes market, which was growing at a moderate rate till 2006-2007, is expected to rise at a skyrocketing pace in the coming years. Hence we can conclude that most of the demands of human, in this and fore coming generation will be fulfilled by carbon nanotubes.
Comments are closed.